
DRUG RePURPOSING PROGRAM
Drug repurposing is an attractive strategy of finding a new usage for existing drugs. It is especially beneficial for rare diseases for which a cure is urgently needed and the process of new drug development is time consuming, expensive, and frequently unsuccessful. Furthermore, with such a small number of patients, pharmaceutical companies have little interest in channeling resources toward research for ultra-rare disease treatments.
Identification of a drug candidate can be made through computational and experimental approaches.
Computational ADSLd drug repurposing
A text-mining AI platform called mediKanren was used to search biomedical datasets for drugs and chemical compounds that could affect pathways disrupted by ADSL deficiency. While no strong candidates for immediate use were found at this time, several compounds were identified that might interfere with PAICS activity (an enzyme of the purine biosynthesis pathway), which could potentially reduce the synthesis and accumulation of a specific toxic metabolite SAICAr (succinylaminoimidazolecarboxamide riboside) (Patent).
However, these compounds have only been identified based on protein structure data, and no experimental studies have been published to confirm their effects on ADSL deficiency.
The evaluation of potential drug repurposing candidates for ADSL deficiency was done at Hugh Kaul Precision Medicine Institute at the University of Alabama at Birmingham and will be repeated as new information on protein structure and function becomes available.
Experimental ADSLd drug repurposing
Animal models and cell lines are used for experimental drug repurposing.
1. Baker’s yeast (Saccharomyces cerevisiae)
The ADSL gene is essential for fundamental cellular processes therefore it is functionally conserved across the animal kingdom, including the genome of the baker’s yeast, Saccharomyces cerevisiae.
S. cerevisiae contains an ADSL ortholog called ADE13. ADE13-deficient or “sick” yeast strains exhibit poor growth (see image below).
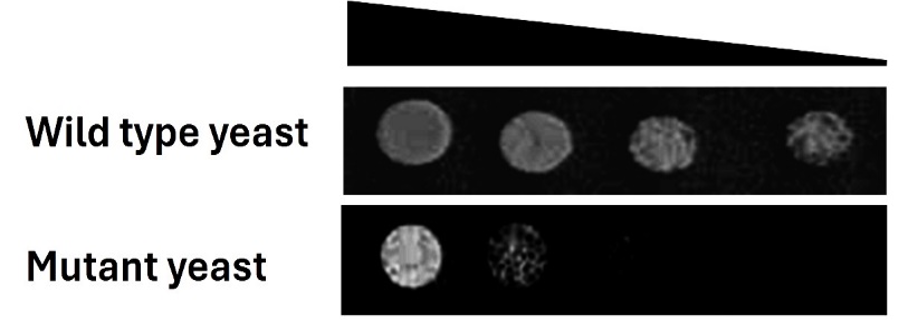
Our objective was to determine if any of the 2,177 compounds from the Pharmakon library (a drug collection) would rescue this poor growth. Upon analysis of the drug repurposing screening data, we identified a “hit” or a promising candidate: disulfiram. This decades-old drug, approved by the FDA in 1951, has been traditionally used for treating chronic alcoholism.
In the initial observational study (n=1), Minja has been treated with a low dose of disulfiram. While regularly checking her biochemical parameters to verify the effect of disulfiram we started collecting Minja’s urine for metabolic profiling. If you would like to learn more about this experiment click here.
Next steps include screening of the TargetMol library, which comprises a substantially larger number of FDA-approved compounds and bioactives (8,387).
2. Roundworm (Caenorhabditis elegans)
After we got “hits” with the experiments with yeast, our goal was to test disulfiram efficacy using another model organism. Dr. Wendy Hanna-Rose from Pennsylvania State University has established the roundworm Caenorhabditis elegans, as an effective model for studying ADSLd. As a multicellular organism with a nervous system, it exhibits a greater functional similarity to humans compared to yeast. Roundworm has a simple and fully identified neural network enabling nervous system function to be studied under conditions of ADSL depletion (Fenton et al., 2017). A decrease in ADSL function in C. elegans results in severe mobility defects and a distinct neurobehavioral response. The effects of disulfiram are currently being analyzed on these well-established learning and locomotion phenotypes in C. elegans. Furthermore, other candidates identified from yeast screening are undergoing the same testing.
While swimming, the ADSL mutant worms are curling up more; they are uncoordinated, slower, and smaller compared to regular, wild type worms. (Video by Wendy Hanna-Rose).
3. Cell lines
Independently, a veteran in the field of ADSL research, Dr. Marie Zikánová from Charles University in Prague, conducted a high-throughput screening (HTS) of potential inhibitors. Disulfiram was shown to inhibit ADSL and PAICS (an enzyme in the purine synthesis pathway). Further experiments using human cell lines with mutated ADSL gene are currently being conducted with no conclusive results yet.
GeNE REPLACeMENT THeRAPY PROGRAM
In addition to drug repurposing, we have initiated a second, more complex project – the development of gene replacement therapy. Its objective is to deliver a functional and therapeutic ADSL gene into the central nervous system (CNS) of ADSL patients like Minja, with the aim to permanently enhance patients’ health.
The gene will be delivered via a nonpathogenic virus called Adeno-associated virus (AAV) whose genes are replaced with a functional copy of the ADSL gene. Thus, due to the natural ability of viruses to infect cells, an ADSL copy will be delivered and a functional ADSL enzyme will be synthesized. See the video here for how AAV gene transfer works. Using AAV9 vectors for CNS gene transfer has become a gold standard in gene therapy. Furthermore, the track record for human application demonstrates a favorable safety record.
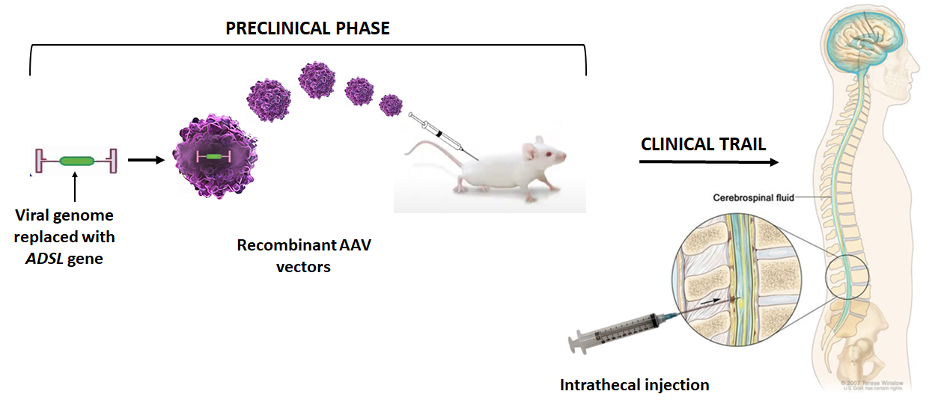
The aim of this project is to design an AAV9 vector to carry the ADSL gene broadly across the CNS in mice. Early proof-of-concept studies will identify the potential efficacy and toxicity of the ADSL gene transfer in mice. If successful, preclinical studies in animals could eventually lead to the application of this approach for treatment in humans.
This project is being conducted at UT Southwestern Medical Center in Dallas, Texas. To learn more about the groundbreaking work of the scientists who pioneered gene therapy treatments for various rare neurological diseases, please visit Gray Lab | UT Southwestern, Dallas, Texas.